Abstract
Introduction Up to 30% of acute myeloid leukemias (AML) evolve from prior hematologic disorders and are classified as secondary AML. Secondary AML is traditionally associated with worse prognosis than de novo, or primary, AML, resulting in inferior response rates and poorer survival. The treatment landscape for AML has evolved recently with the introduction of venetoclax (VEN) and the availability of targeted agents. To better understand evolution of the management of secondary AML, we evaluated treatment patterns and outcomes in patients (pts) with primary AML vs secondary AML treated in real-world clinical practice and enrolled in the CONNECT® Myeloid Disease Registry (NCT01688011) by type of site (academic or community/government).
Methods Pts were classified as having either primary or secondary AML based on history of myelodysplastic syndrome (MDS) or progression from MDS to AML. Overall survival (OS) from the date of AML diagnosis, estimated by the Kaplan-Meier method, was evaluated in each group according to type of site (academic or community/government), utilization of allogeneic hematopoietic stem cell transplantation (alloHSCT), and tumor protein p53 (TP53) mutational status. Time to adoption of a VEN-based therapy, defined as first use of VEN-based therapy following Food and Drug Administration (FDA) approval in this setting, was evaluated using a Cox model with factors for age (< 75 and ≥ 75 y), type of site, and geographic region.
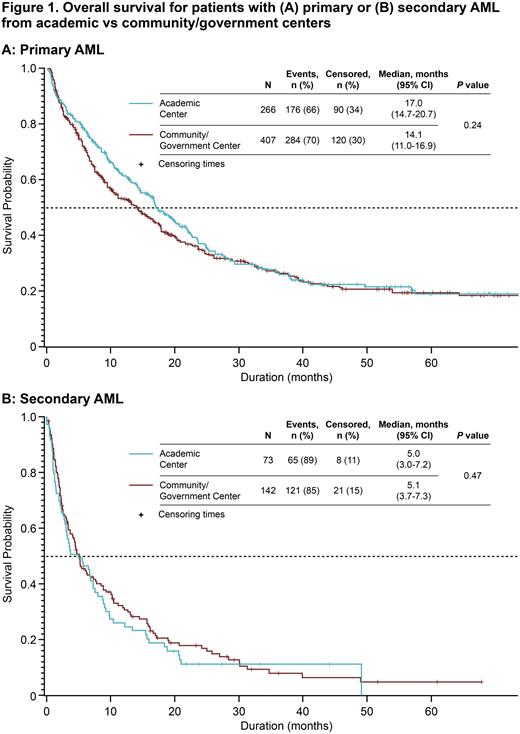
Results A total of 891 pts in the Registry with a diagnosis of AML were assessed (primary AML, n = 676; secondary AML, n = 215). Median (range) ages were 70 (55-97) y in the primary AML group and 74 (19-93) y in the secondary AML group. Most pts were male (primary AML, 61.6%; secondary AML, 61.5%) and most were treated at community or government centers (primary AML, 60.2%; secondary AML, 66.0%). Pts with primary AML had significantly longer survival than pts with secondary AML; median OS (95% confidence interval [CI]) was 15.6 (13.6-17.4) mo and 5.1 (3.6-6.7) mo, respectively (P < 0.001). Median OS for pts with primary AML and secondary AML treated at academic centers was 17.0 mo and 5.6 mo, respectively (hazard ratio [HR] [95% CI], 0.41 [0.30-0.54], P < 0.001) and in community/government centers was 14.1 mo and 5.2 mo, respectively (HR [95% CI], 0.53 [0.43-0.66], P < 0.001). There was no significant difference in median OS in pts treated at academic centers compared with pts treated at community/government centers for both primary AML (academic vs community/government centers: 17.0 mo [95% CI, 14.7-20.7] vs 14.1 mo [11.0-16.9]; Figure 1A) and secondary AML (5.0 mo [95% CI, 3.0-7.2] vs 5.1 mo [3.7-7.3]; Figure 1B). Significantly longer median OS in pts with primary AML vs secondary AML was observed regardless of alloHSCT status (no alloHSCT: 11.7 mo vs 4.5 mo; HR [95% CI], 0.53 [0.44-0.64], P < 0.001; with alloHSCT: 64.3 mo [35.8-NC] vs 24.9 mo [6.8-48.9], P = 0.002]. Longer median OS was observed among pts who received alloHSCT compared with those without alloHSCT: primary AML, 41.1 mo vs 12.8 mo; secondary AML, 24.9 mo vs 4.4 mo; additional analyses are warranted to account for immortal time bias. Regardless of TP53 mutation status, pts with primary AML had significantly longer median OS than pts with secondary AML (no mutation: 22.0 mo [17.6-26.1] vs 6.2 mo [2.5-12.6], P < 0.001; with mutation: 8.8 mo [3.9-14.2] vs 3.8 mo [1.4-6.8], P = 0.014). VEN-based therapies were adopted more quickly by pts aged ≥ 75 y vs < 75 y (P < 0.001) and those treated at community or government centers vs academic centers (P = 0.045); no association between geographic region and time to adoption of VEN-based therapies was observed.
Conclusions Overall, outcomes of pts with AML in the CONNECT® Myeloid Disease Registry are consistent with previously reported outcomes. Median OS is longer in primary AML vs secondary AML regardless of whether pts receive alloHSCT or have mutations in TP53. This analysis suggests that contrary to commonly held perceptions, site of care does not affect overall pt outcome and that VEN adoption occurs sooner at community centers. The clinically meaningful increase in OS in pts receiving alloHSCT, when compared with pts without alloHSCT, regardless of primary or secondary AML further emphasizes the importance of providing pts with the opportunity for transplant. These observations merit further study into potential differences in treatment patterns and outcomes.
Disclosures
Scott:Novartis: Other: Advisory Panel, Research Funding; Alexion: Consultancy; Jazz Pharmaceuticals: Other: Advisory Panel; Nektar: Other: data and safety monitoring board; Johnson and Johnson: Other: data and safety monitoring board; Incyte: Consultancy; Celgene: Consultancy, Honoraria, Other: Advisor Panel; Bristol Myers Squibb: Consultancy, Honoraria, Other: Advisory Panel, Research Funding. Savona:AbbVie: Consultancy, Other: travel expenses; Novartis: Consultancy; ALX Oncology: Research Funding; Takeda: Consultancy; Forma: Consultancy; Astex Pharmaceuticals: Research Funding; Sierra Oncology: Consultancy, Other: travel expenses; Karyopharm Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Other: Travel expenses, Research Funding; Geron: Consultancy; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Taiho Pharmaceutical: Consultancy; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; Incyte Corporation: Research Funding. Roboz:Amgen: Consultancy; Pfizer: Consultancy, Honoraria, Other: Travel and accommodation expenses; Novartis: Consultancy, Other: Travel and accommodation expenses, Research Funding; Jazz: Consultancy, Other: travel; MEI Pharma: Consultancy, Research Funding; Otsuka: Consultancy; Bristol Myers Squibb: Consultancy; Roche: Consultancy; Helsinn Therapeutics: Consultancy; Array BioPharma: Other: Travel and accommodation expenses; Amphivena Therapeutics: Other: Travel and accommodation expenses, Research Funding; Mesoblast: Consultancy; CTI: Research Funding; Karyopharm Therapeutics: Research Funding; Mofitt Cancer Center: Research Funding; Amgen: Consultancy, Other: travel; Astex Pharmaceuticals: Consultancy, Other: Travel and Accommodation expenses, Research Funding; Eisai: Other: Travel and accommodation expenses; Sunesis Pharmaceuticals: Other: Travel and accommodation expenses, Research Funding; Celltrion: Consultancy, Other: Travel and accommodation expenses; Takeda: Consultancy; Onconova Therapeutics: Research Funding; Clovis Oncology: Other: Travel and accommodation expenses; Agios: Other: travel, Research Funding; Genentech/Roche: Consultancy, Other: Travel and accommodation expenses; Bayer: Consultancy, Other: Travel and accommodation expenses; Daiichi Sankyo: Consultancy; Sandoz: Consultancy, Other: Travel and accommodation expenses; MedImmune: Consultancy, Research Funding; Jasper Therapeutics: Consultancy; Janssen: Consultancy, Other: travel and accommodation expenses, Research Funding; GlaxoSmithKline: Consultancy; Agios: Consultancy, Research Funding; Celgene: Consultancy, Other: travel and accommodation expenses, Research Funding; Astellas: Consultancy; Bristol Myers Squibb: Consultancy; Tensha Therapeutics: Research Funding; AbbVie: Consultancy, Other: travel and accommodations, Research Funding; Actinium: Consultancy. Seiter:Sellas Life Sciences: Research Funding; Rafael Pharmaceuticals: Research Funding; Alexion Pharmaceuticals: Honoraria; Novartis: Honoraria; Incyte: Honoraria, Research Funding; Jazz Pharmaceuticals: Research Funding; Theradex: Research Funding; GlycoMimetics: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding. DeGutis:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kiselev:Celgene: Current holder of stock options in a privately-held company; Juno: Current holder of stock options in a privately-held company; Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Yu:BMS: Current Employment, Current equity holder in publicly-traded company, Other: travel and accommodation expenses. McBride:Bristol Myers Squibb: Current Employment. Heydendael:Bristol Myers Squibb: Current Employment. Erba:Daiichi Sankyo: Consultancy, Research Funding; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; MacroGenics: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Covance (Abbvie): Consultancy, Other: Independent Review Committee, Research Funding; Janssen Oncology: Consultancy; Takeda: Consultancy; Trillium Therapeutics: Consultancy; Astellas Pharma: Consultancy; Abbvie: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Glycomimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ImmunoGen: Consultancy, Research Funding; Celgene: Consultancy, Other, Speakers Bureau; Amgen: Consultancy, Research Funding; Agios: Consultancy, Research Funding, Speakers Bureau; Kura Oncology: Consultancy; Forma Therapeutics: Research Funding; Gilead/Forty Seven: Research Funding; PTC therapeutics: Research Funding; ALX Oncology: Research Funding; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal